 |
 |
|
||||||||||||||||||||
|
|
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
 |
|
||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
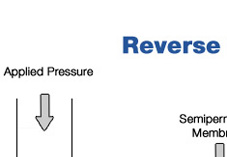
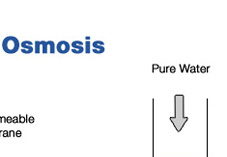
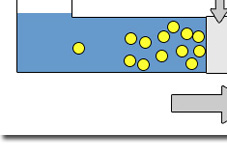
About Reverse Osmosis Reverse Osmosis is the process by which water is forced through a semi-permeable membrane; a material with microscopic pores in it. Purified water passes through the membrane while viruses, bacteria, parasites (giardia, cryptosporidium), carcinogens, herbicides, pesticides, radioactive contaminants, sulfates, aluminum, arsenic, cadmium, iron, lead, zinc, chloramine, trihalomethane, fluoride, furan and more are left behind and flushed down the drain. The key to remember about osmosis is that water flows from the solution with the lower solute concentration into the solution with higher solute concentration. With the application of pressure, the process can be reversed. This process is called reverse osmosis. The procedure was developed in the late 1950's and 1960's partly to fulfill the needs of the emerging Space Program. An early application of reverse osmosis was in the space capsule, where it was required that drinking water be processed from waste water onboard the spacecraft. Later development in membrane technology make reverse osmosis water treatment practical for the home. Today, an important application of reverse osmosis is in the production of drinking water from sea water called desalination. Most bottled water companies use reverse osmosis to make the "premium pure water" that they deliver to your home or office. Kidney dialysis machines are another application of this type of process. Read our FAQ & White Paper |
|
|
|||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||
|
|
 |
|
|
|||||||||||||||||||
 |
 |
|
||||||||||||||||||||
 |
 |
|
||||||||||||||||||||
 |
|
|||||||||||||||||||||
 |
|
|
|
|
|
|
 |
|
||||||||||||||
 |
|
|||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|